4 ways to accelerate the development of medical implants with design automation

Written by nTop
Published on October 26, 2020
A major factor that influences design decisions during the product development of medical devices is time-to-market. Accelerating or automating your design processes can give you a distinct advantage over your competitors. Here are four medical device videos that show you how you can achieve this with nTop. We recommend you to bookmark this page for your future reference.
Applications
Key Software Capabilities
- Lattice structures
- Design automation
Ensuring traceability
First up we’ve got, Manage and trace design variations for large product families and DoE. In this nTop Live, Christopher Cho, Staff Application Engineer at nTop, shows how to modify your workflows in nTop to improve design traceability.
Watch and learn how to:
- Organize your work using variables for important input parameters
- Automatically assign a unique filename to design outputs during export
- Create repeatable design workflows that produce traceable results
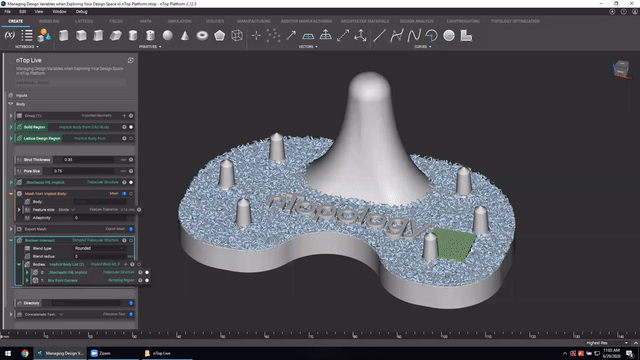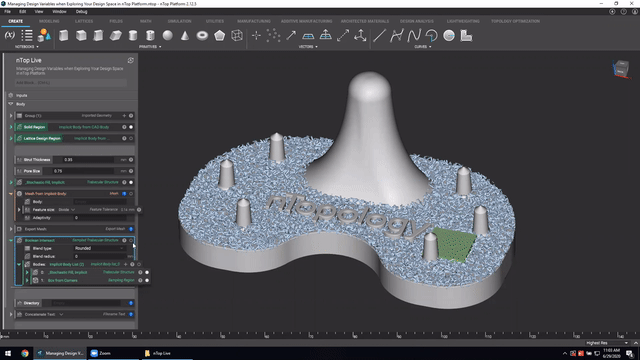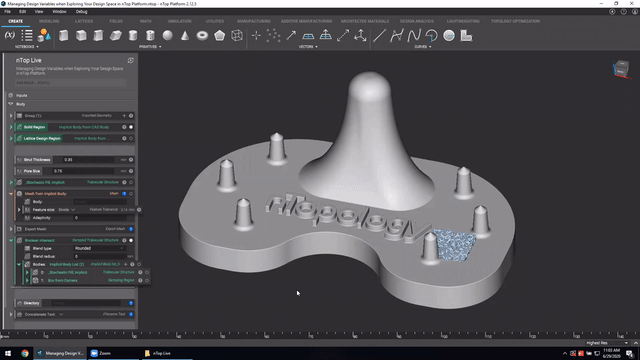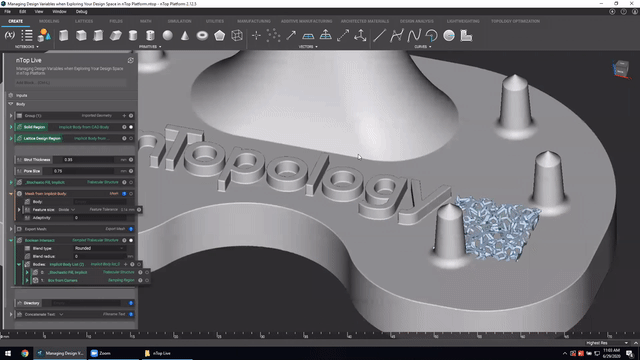
Screenshot from Manage and trace design variations for large product families and DoE video
Next, we’ve got Manage and trace key design properties through automatic report generation. Medical device designers are well aware that a traceable design process is not only required for regulatory compliance but also improves the credibility of your product(s) and ensures corrective and preventive actions will take place should something go wrong.
Check out this video to see how to automatically generate a report file in nTop.
Watch and learn how to:
- Document part properties in a file to ensure design traceability
- Run automated reporting workflows in parallel to your design workflows
- Enhance existing design workflows and improve your quality-of-life
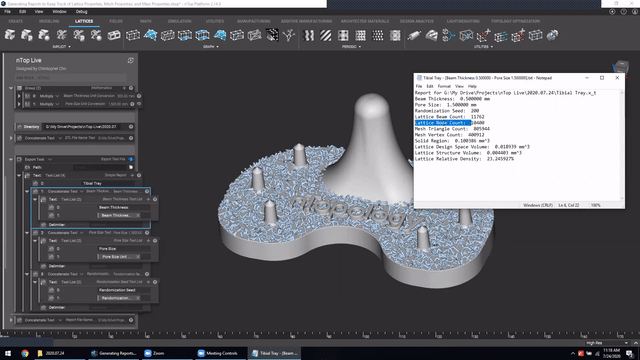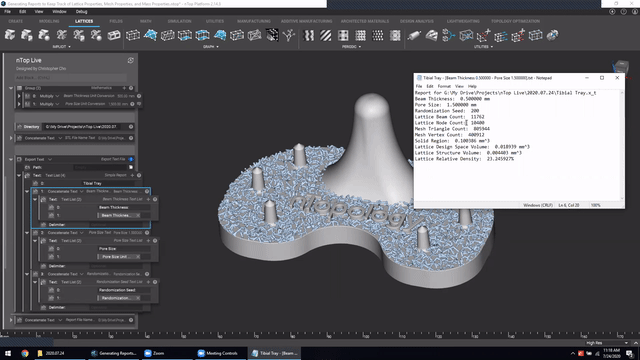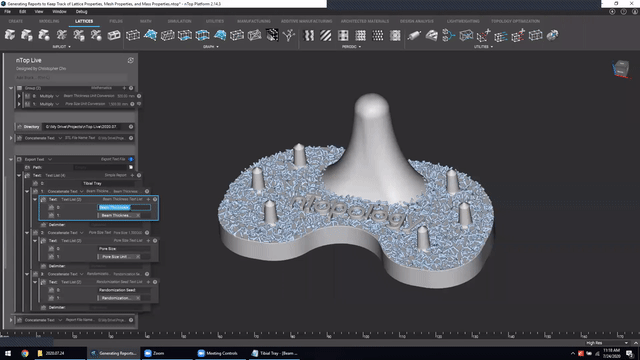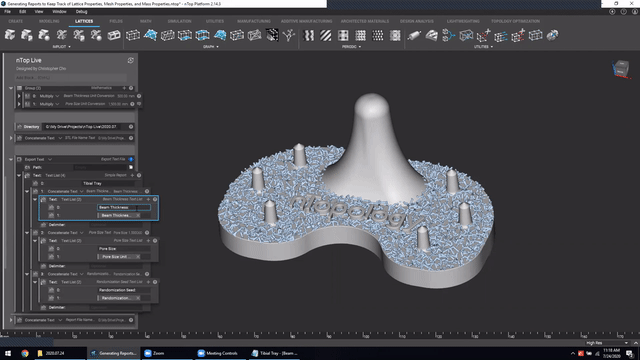
Screenshot from Manage and trace key design properties through automatic report generation video
Managing product families
As a product line matures, key design features tend to get standardized and reused. To maintain and expand large product families, robust design automation workflows can greatly accelerate your development cycles.
In this video, Reusable design workflows for large product families of medical devices, we demonstrate what a mature design workflow can look like for a large product family in nTop.
Watch and learn how to:
- Reuse the same design workflow on different part configurations
- Maintain, expand, and trace a large product family
- Batch process multiple parts using the same nTop Workflow
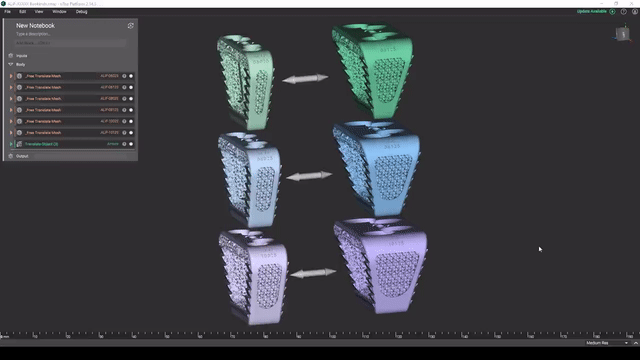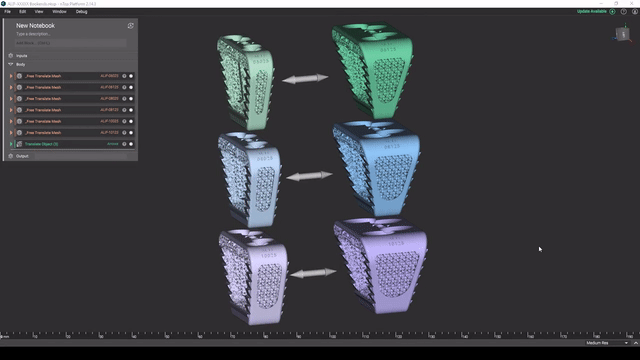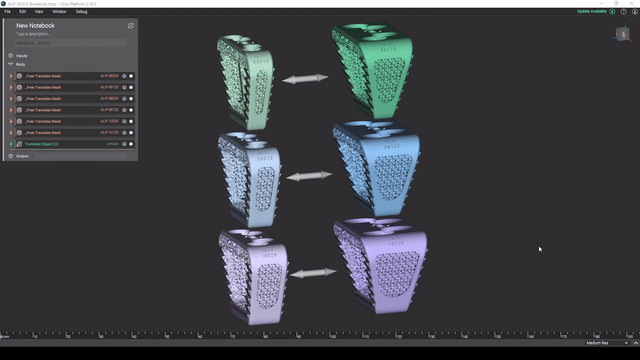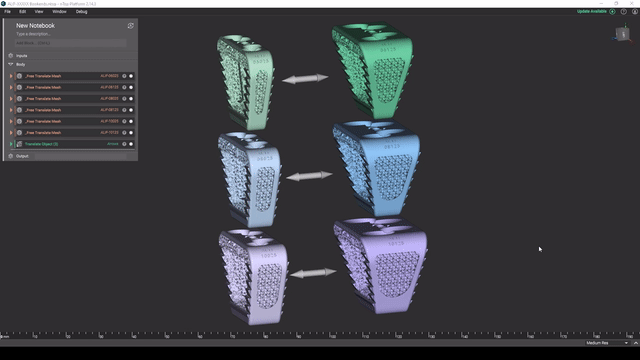
Screenshot from Reusable design workflows for large product families of medical devices video
Designing medical implants
Last, we’ve got Accelerated design of patient-specific implants. We go over how to leverage patient-specific geometry as an input to build conformal bone plates.
Watch this video to learn how to:
- Design patient-specific implants from existing 3D data
- Automatically generate mounting features
- Work more efficiently with patient-specific data
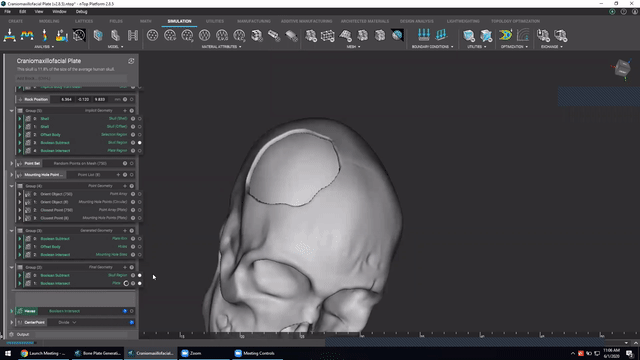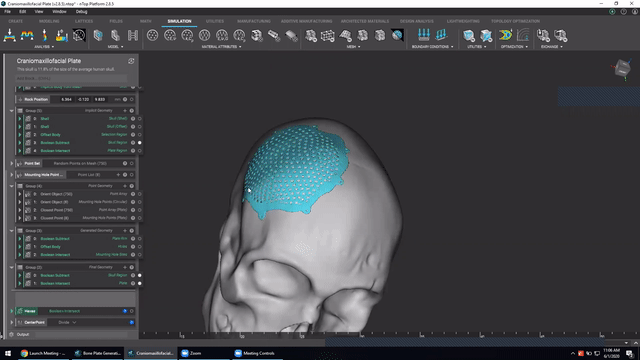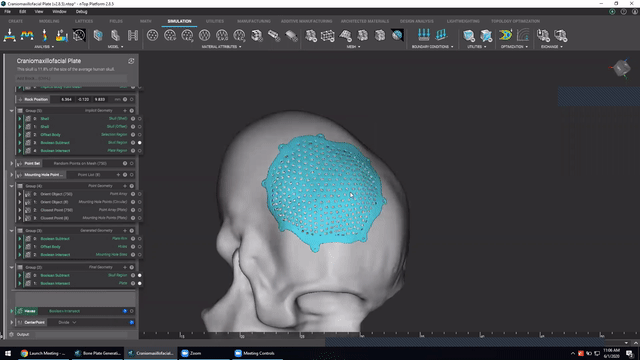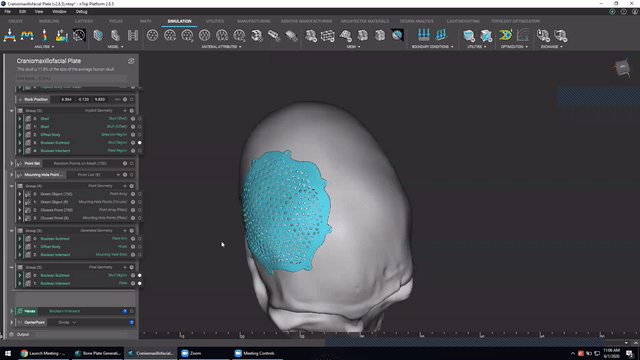
Screenshot from Accelerated design of patient-specific implants video

nTop
nTop (formerly nTopology) was founded in 2015 with the belief that engineers’ ability to innovate shouldn’t be limited by their design software. Built on proprietary technologies that upend the constraints of traditional CAD software while integrating seamlessly into existing processes, nTop allows designers in every industry to create complex geometries, optimize instantaneously, and automate workflows to develop breakthrough 3D-printed parts in record time.
Related content
- VIDEO
Thermal Applications - The Hot Topics

- VIDEO
Design better implants for osseointegration with the Lattice Pore Size Block

- VIDEO
nTop CDS 2024: Exploring the cutting edge of computational design with Matthew Shomper

- VIDEO
nTop CDS 2024: How COBRA Golf created the LIMIT3D irons

- CASE STUDY
Cobra Golf designed their LIMIT3D irons 50% faster with nTop
