3D printing implants: A complete guide
Written by nTop
Published on February 1, 2023
This article explores how 3D printing is changing the way we design and manufacture medical implants. You will learn how you can achieve the full benefits of this new technology by embracing a digital fabrication workflow.
Personalized medical devices, including 3D-printed implants, are becoming more popular. These implants match patients' unique physiology and anatomy, providing a superior fit and enabling faster healing times.
While patient-specific implants still account for only a small portion of the overall market, they will continue to increase in popularity as advances in 3D printing and other technologies make them more widely available.
This article explores how the rise of 3D printing is making patient-specific implants more accessible, the benefits of leveraging a digital workflow to design 3D-printed implants, and important considerations you will need to take into account along the way.
Additive manufacturing and implants
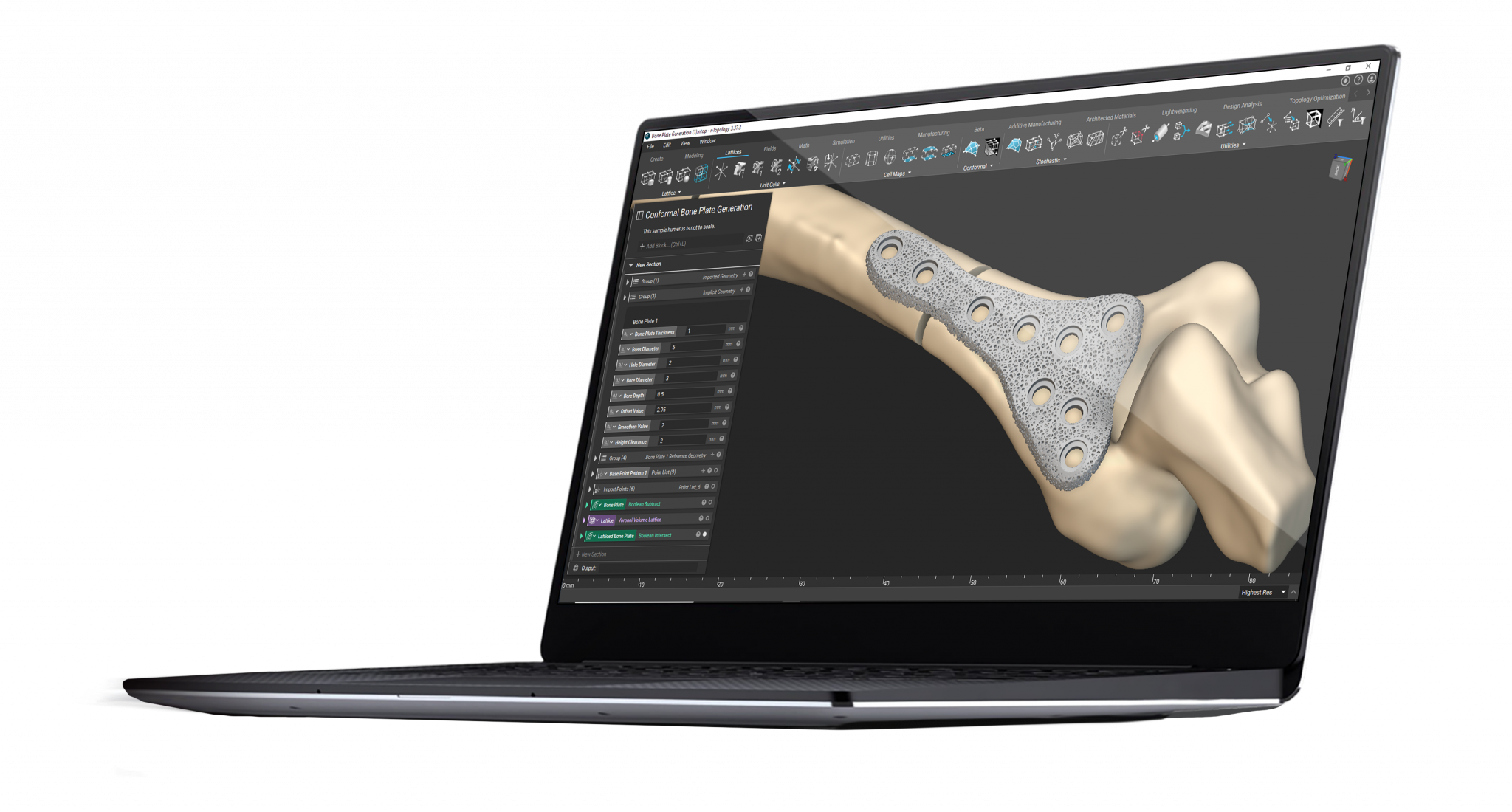
Anterior Lumbar Interbody Fusion (ALIF) spinal implants being designed in nTop.
Additive manufacturing (AM) is not a new technology in developing medical implants. For the last decade, 3D printing has enabled implant manufacturers to create complex geometries that mimic the function and shape of natural bone. But the role of 3D printing in medical implants is evolving from an unconventional manufacturing method into an avenue for reshaping what implants are capable of and how medical professionals can treat patients.
One of the primary advantages of 3D printing is that it enables accelerated product development. This is because 3D printing allows you to create new geometries, like trabecular lattices that encourage bone in-growth, and rapidly prototype these geometries using the intended manufacturing process. After testing and design finalization, the implant can go into production.
This accelerated product development process means you can develop patient-specific implants significantly faster, reducing patient wait time and promoting better health outcomes.
3D printing technology also offers new ways of working with common implant materials, creating opportunities for biocompatibility. Although additive manufacturing offers a relatively limited range of materials, you can overcome this limitation with intelligent design and by taking advantage of AM's ability to manufacture complex structures.
Some of the available biocompatible materials for 3D printing include PEEK, Titanium, and Nylon. However, the range of biocompatible and sterilizable materials is still limited and remains an active research area.
Another critical benefit of 3D-printed, patient-specific implants is that they can simplify procedures. Many reconstructive procedures require a significant amount of artistry and manual handiwork for the surgeon. Working with patient-specific implants, made possible by AM, means procedures can be faster and less invasive, accelerating patient recovery times and improving outcomes.
Workflow for 3D printing implants
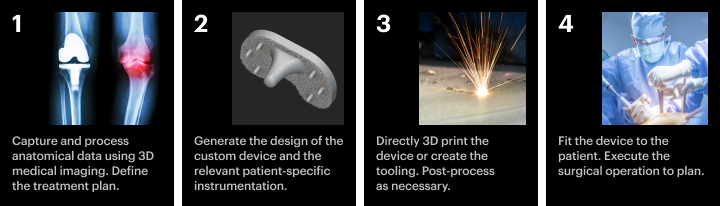
The digital workflow for 3D printing implants.
The digital workflow for 3D printing implants varies depending on the specific use case, but the basic steps remain the same.
1. Patient data acquisition
First, the healthcare provider will capture high-resolution anatomical data using 3D medical imaging technologies, like MRIs, CT scans. This data is then segmented and converted into digital surface meshes used in downstream design operations. At this stage, clinicians may provide additional patient data that could inform the treatment plan, such as anatomical landmarks.
2. Design generation
Depending on your customization strategy and the use case, you can generate the implant's design with different levels of automation. For example, you could rely on one-off manual designs or fully automate the design of patient-matched implants.
You may also need to design custom instrumentation, such as surgical guides, at this stage. Remember, the design process is as important as the design output because it ensures compliance with medical protocols.
3. Manufacturing
You can leverage 3D printing to produce your implant directly or indirectly. The indirect method involves creating custom tooling to support the primary manufacturing process. While the indirect approach scales better when working with large production volumes, the direct approach is beneficial when creating implants with design features only possible with AM.
In both cases, you will need to subject the implant to specific post-processing after manufacturing to achieve the necessary functional requirements, such as sterilization.
4. Delivery to the patient
Because the implant matches the patient's physiology and anatomy, they require less effort to implant than stock devices. This is because stock devices often need adjustments in the operating room, causing complications that increase the medical practitioner's workload.
Use cases
3D-printed medical implants are being used more frequently in many medical applications for orthopedic, reconstructive, and plastic surgery.
Implants for orthopedic surgery
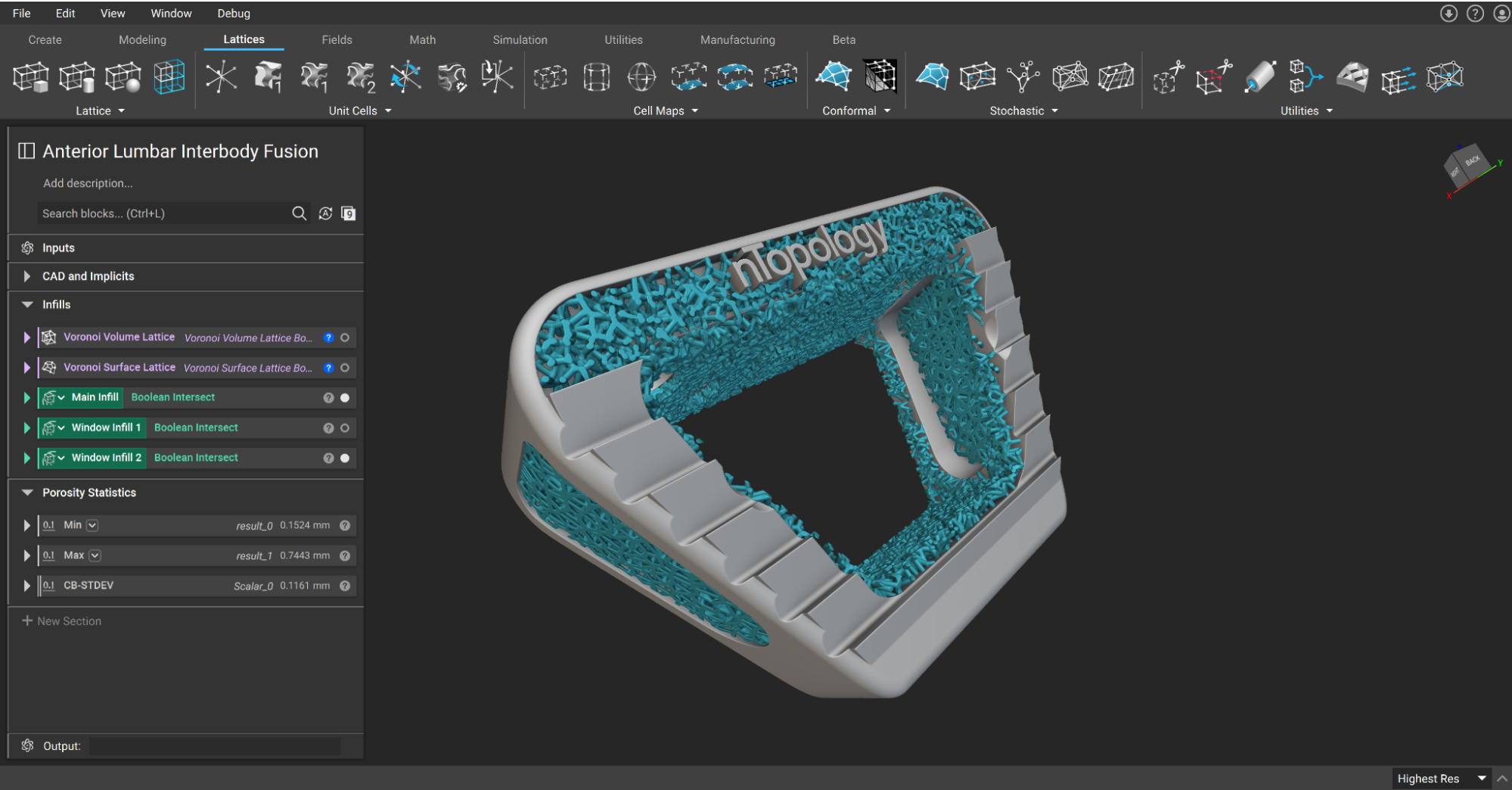
ALIF spinal implant featuring porous trabecular structures for cementless fixation.
Examples of personalized orthopedic implants include bone plates, spinal implants, and joint replacements for the hip, shoulder, and knee. These personalized devices offer better alignment and coverage and can also feature trabecular structures that match the bone's mechanical properties, improve fixation, and promote osseointegration. Osseointegration is the direct functional and structural connection between the surface of the implant and the living bone, which enhances implant stability.
Implants for reconstructive and plastic surgery
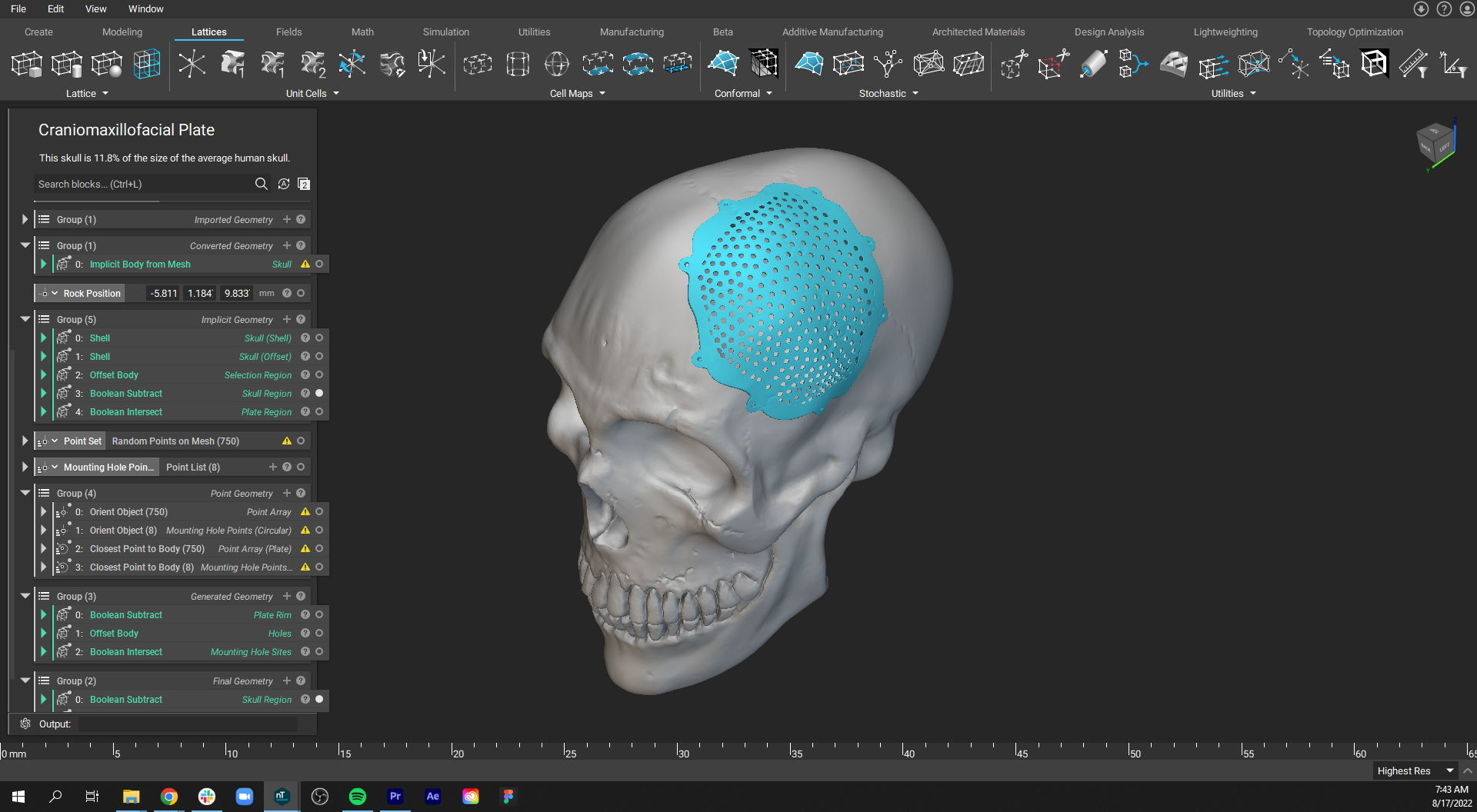
Patient-specific cranial bone plate with shape that mirrors the undamaged part of the skull.
3D-printed medical implants for reconstructive and plastic surgery include cranial and maxillofacial implants and bone grafts. Patient-specific implants in this field offer excellent anatomical fit and enable complete restoration and aesthetic results while reducing costs associated with postoperative infections.
Surgical guides
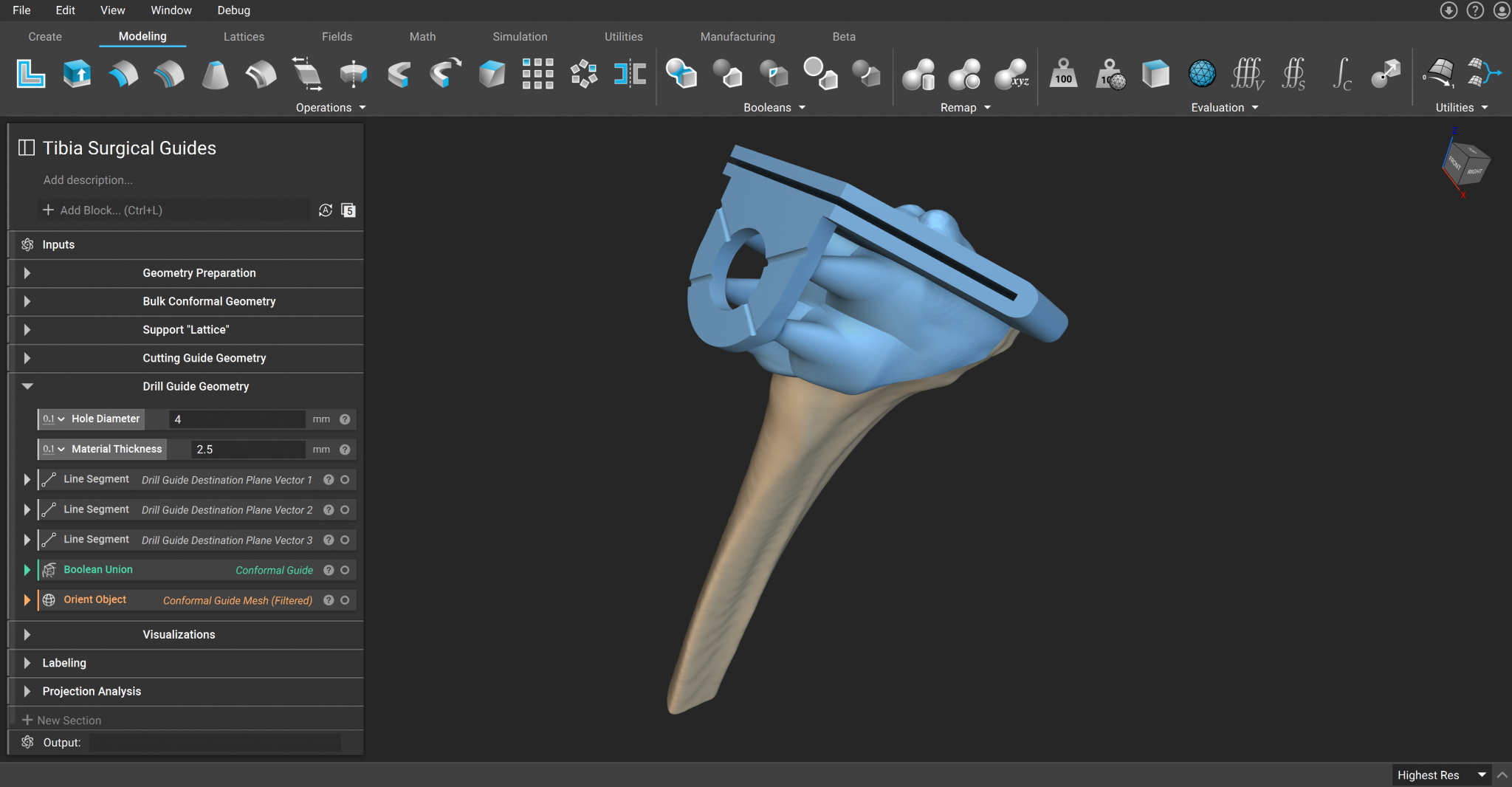
A custom surgical guide that conforms to tibia contour to improve cutting precision.
3D-printed surgical guides, like cutting guides and patient-specific instrumentation, are becoming increasingly common. These devices support the implantation process and can decrease medical errors, shorten surgery times, and mitigate the likelihood of revision surgery being necessary.
Key considerations when designing 3D-printed implants
The design and manufacture of medical implants using 3D printing technology can offer many benefits to patients, such as improved functionality, increased biocompatibility, and shorter production times. However, to maximize the benefits of 3D-printed implants, you will need to consider the following:
Lattices and osseointegration
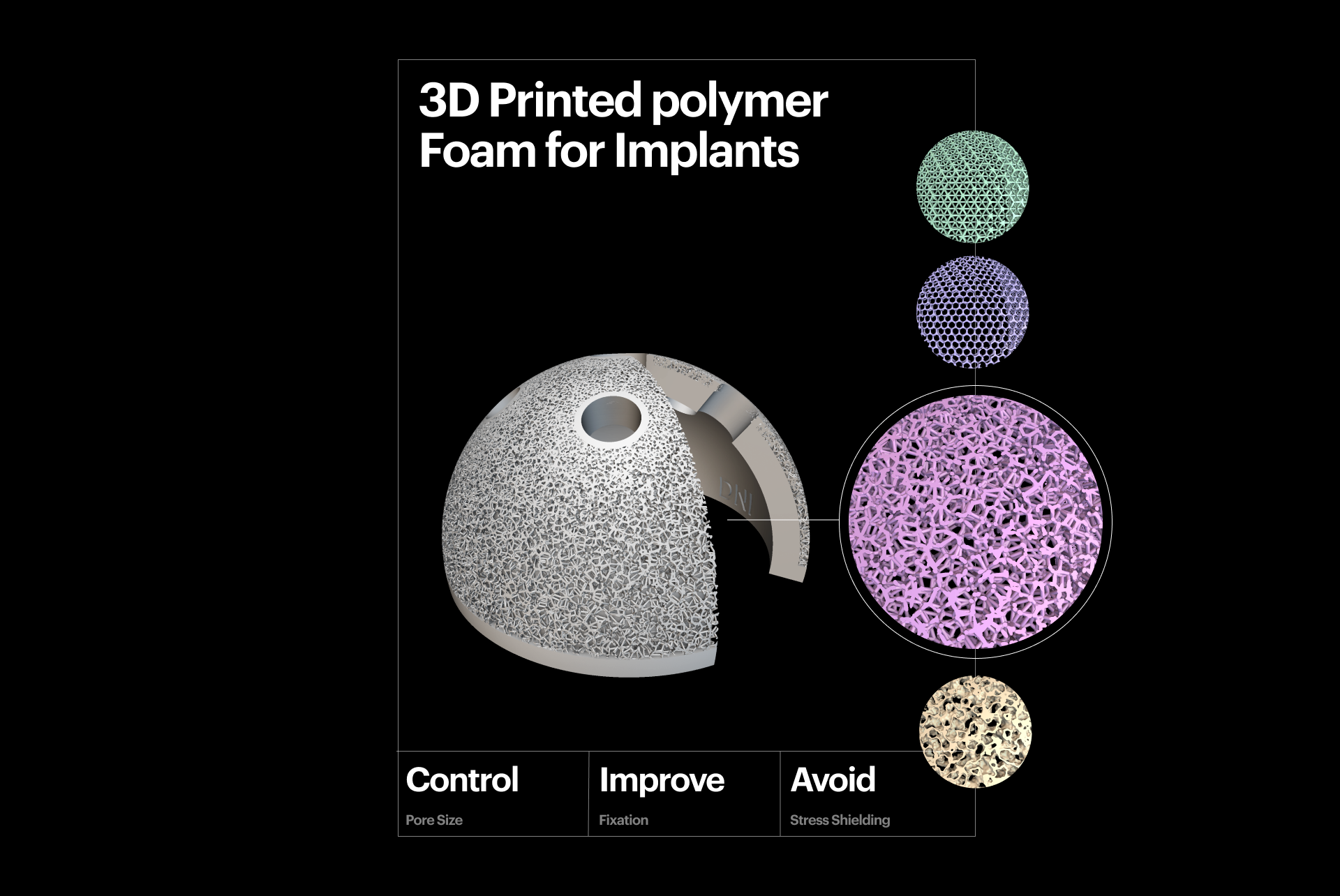
3D-printed polymer foam for implants.
One consideration is the lattice or porous structure used in the implant and how effectively it can promote osseointegration.
Additively manufactured implants often feature porous metal foams, allowing you to fine-tune porosity, pore size, shape, and distribution to improve osseointegration, as shown in both in vivo and in vitro clinical studies. These implants don't require cement and often reduce the time spent in the operating room by 25%.
Regulatory compliance
Regulatory approval is essential to ensure patient safety and improve patient outcomes, but achieving compliance can be complex. In addition to the usual validation hurdles, developing patient-specific implants requires you to adhere to the same quality control standards even as the geometry changes.
If your device includes adjustable features, you must ensure that these features can only vary within a pre-determined design window. And when you reach the qualification stage of the approval process, you must be able to demonstrate that each of your devices is within these pre-determined limits.
As regulatory bodies become more familiar with personalized medical devices, getting approval will likely become more manageable.
Ensuring your design process is traceable is crucial in receiving regulatory approval for your patient-specific implants. A traceable design process involves maintaining a digital trail of every design decision and the input parameters that define your device.
Regulatory bodies require that your design process is traceable to grant approval. Other benefits of a traceable design process include improving your implant's credibility, mitigating development risks, and supporting you in taking corrective action when necessary.
Mechanical biocompatibility
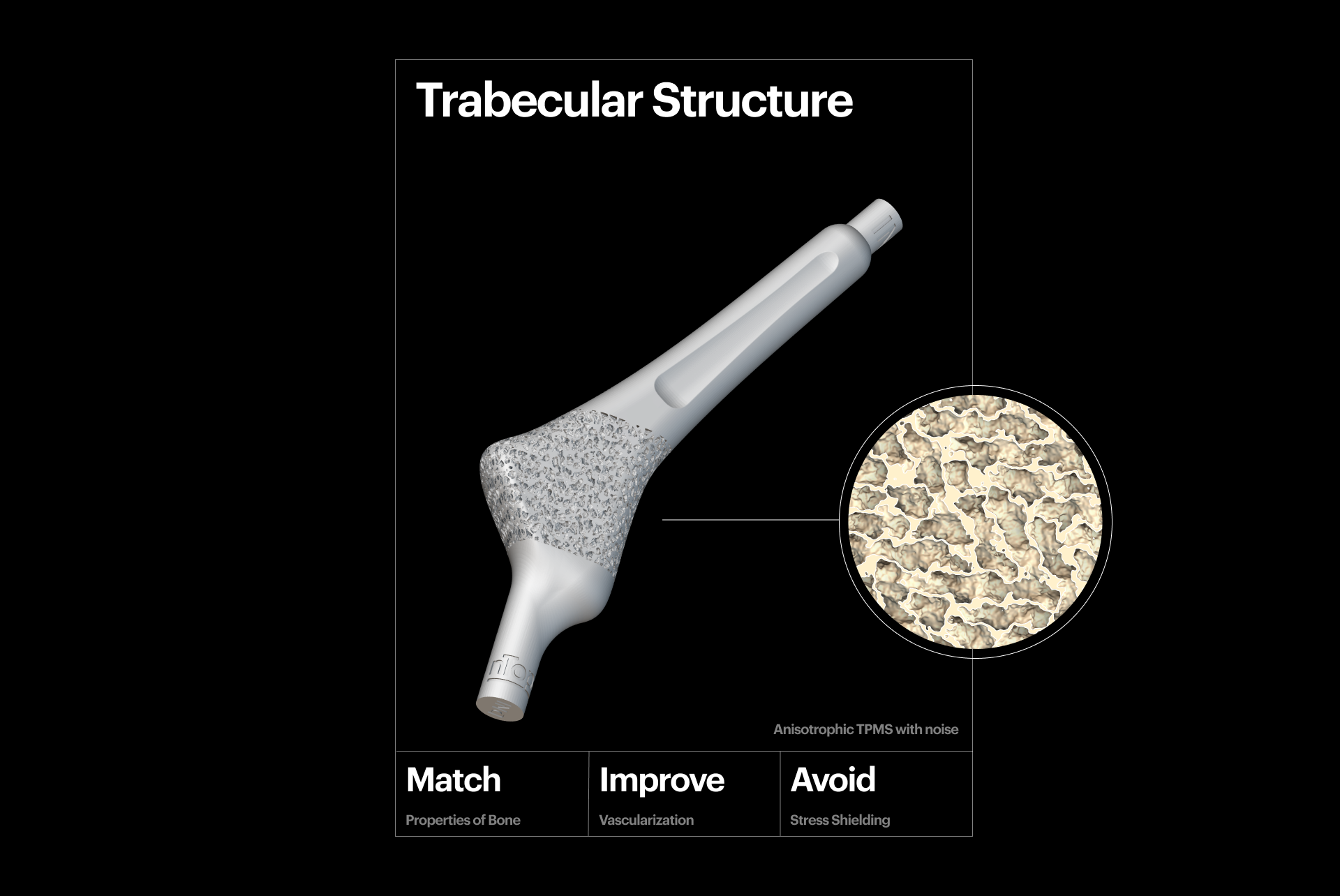
3D-printed trabecular structure.
Mechanical biocompatibility is critical for successful integration with the patient's body. To achieve this and avoid stress shielding, the mechanical properties of your implant, such as Young's modulus and compressive strength, must match the bone's properties. Porous metal scaffolds are excellent for facilitating biocompatibility and can be created using stochastic, TPMS, and graph lattice structures. These structures are especially effective because you can precisely tune and locally optimize their properties to achieve the desired effect and mitigate the risk of implant failure.
Case study: SI-BONE

Implantable orthopedic devices by SI-BONE, designed in nTop.
SI-BONE is a medical device company that has designed a novel osseointegrative lattice structure used in minimally-invasive joint surgery. This innovative structure offers better support for bone fusion.
The SI-BONE engineering team applied this lattice structure to a product family with more than 70 unique parts. The team achieved this in less than six hours while ensuring the traceability of the design process. This new design's key benefits are reduced operational risk and significant time savings. Another advantage is that the design is more reliable and can be reproduced accurately and quickly.
This new technology offers great potential for many companies in the medical device industry, and the FDA awarded it the Breakthrough Medical Device designation.
Software for designing 3D-printed implants

Implant being designed in nTop.
With the maturation of additive manufacturing, there is a rising demand for software that can take full advantage of its design freedom. nTop is a next-generation design software that enables engineers to create innovative, patient-specific implants that maximize the benefits of additive manufacturing.
With nTop, you can quickly and easily design custom medical devices that meet the unique needs of your patients. Find out more about personalized medical devices in our comprehensive guide.
Key takeaways
- While they still account for only a small portion of the market, patient-specific implants will continue to increase in popularity as advances in 3D printing and other technologies make them more widely available.
- There are several challenges associated with 3D printing patient-specific implants, including achieving regulatory compliance, mechanical biocompatibility, and facilitating osseointegration.
- Advanced engineering design software like nTop can help you overcome these challenges and radically improve your 3D-printed implant designs.

nTop
nTop (formerly nTopology) was founded in 2015 with the belief that engineers’ ability to innovate shouldn’t be limited by their design software. Built on proprietary technologies that upend the constraints of traditional CAD software while integrating seamlessly into existing processes, nTop allows designers in every industry to create complex geometries, optimize instantaneously, and automate workflows to develop breakthrough parts and systems in record time.
Related content
- CASE STUDY
Replacing spacecraft supermaterial with high-performance lattice

- VIDEO
Design better implants for osseointegration with the Lattice Pore Size Block

- VIDEO
nTop CDS 2024: Exploring the cutting edge of computational design with Matthew Shomper

- CASE STUDY
Cobra Golf designed their LIMIT3D irons 50% faster with nTop

- GUIDE
Download: Advanced design software and additive manufacturing for personalized implants
